Emergency Use Authorization (EUA)
What is an Emergency Use Authorization (EUA?)
During a public health emergency, the FDA can use its Emergency Use Authorization (EUA) authority to allow the use of unapproved medical products, or unapproved uses of approved medical products, to diagnose, treat, or prevent serious or life-threatening diseases when certain criteria are met.
The secretary of Health and Human Services must make a declaration of emergency or threat justifying the authorization of emergency use for a product. When the emergency is over, the EUA declaration is terminated, and all EUAs issued based on the declaration will no longer remain in effect. Manufacturers are encouraged to pursue premarket submissions through the appropriate regulatory pathway (e.g., 510(k)) during the emergency so that devices can remain on the market after the emergency.
What products are receiving and subject to EUA during the COVID-19 Pandemic?
The following products are receiving EUAs:
Personal Protective Equipment (PPE)*
In Vitro Diagnostics
Ventilators and Other Medical Devices
*As of May 22, 2020, EUAs are being waived for ‘gowns and other apparel’ in response to concerns relating to insufficient supply and availability of PPE for use by healthcare personnel (HCP). For a complete list and description of gowns and other apparel, please review this document
Where can I find a list of devices with EUA?
The FDA website maintains an up-to-date list of current and terminated EUAs. Please see here Coronavirus Disease 2019 (COVID-19) EUAs for Medical Devices.
More Information:
Emergency Use Authorizations (FDA)
FAQs on EUA for Medical Devices During the COVID-19 Pandemic
COVID-19 EUAs for Medical Devices
Letter of Authorization - Manufacturers of Gowns and Other Apparel (FDA)
Personal Protective Equipment (PPE)
What is PPE?
PPE refers to protective clothing, helmets, gloves, face shields, goggles, facemasks, and/or respirators or other equipment designed to protect the wearer from injury or the spread of infection or illness.
What are the CDC recommendations regarding PPE?
The CDC recommends PPE should be used by:
Patients with confirmed or possible SARS-CoV-2 Infection (should wear a face mask)
Healthcare Personnel should adhere to Standard and Transmission-based Precautions when caring for patients with SARS-CoV-2.
On April 8, 2020 the AMA updated its Code of Medical Ethics (Opinion 8.3) to state that physicians have a well-recognized duty to provide care during a public health emergency and corresponding duty on the part of health care institutions to support and protect their staff.
The Occupational Safety and Health Administration (OSHA) states that employers should adapt infection control strategies based on a thorough hazard assessment, using appropriate combinations of engineering and administrative controls, safe working practices, and PPE to prevent worker exposures and disease spread.
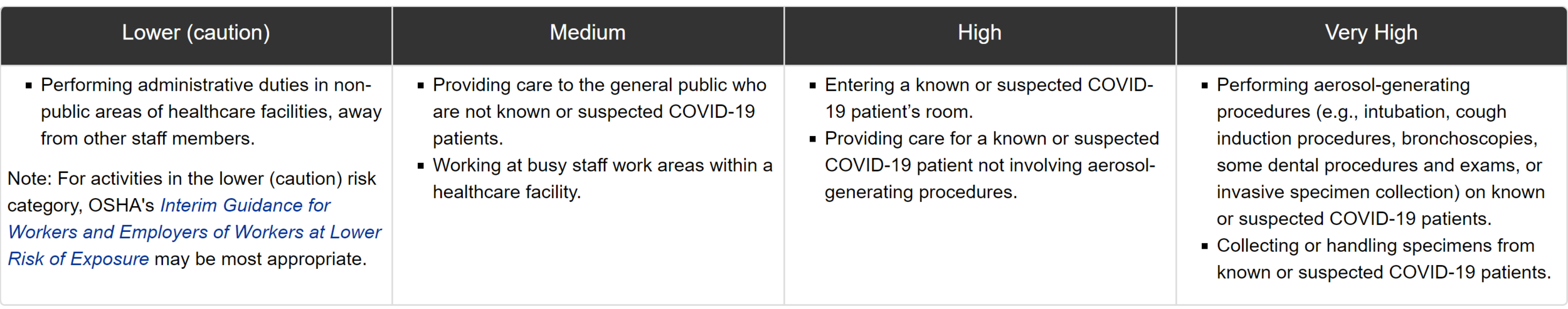
Examples of healthcare work tasks associated with exposure risk levels:
Until more is known about how COVID-19 spreads, OSHA recommends using a combination of standard precautions, contact precautions, airborne precautions, and eye protection (e.g., goggles, face shields) to protect healthcare workers.
When handling specimens and running tests, what PPE should be donned by employees?
The CDC recommends (at a minimum) to don:
Eye protection and/or full splash guard
Disposable, clean nitrile gloves
N95 respirator (or face mask)
Disposable isolation gown or lab coat*
*Lab coats and isolation gowns must be discarded before exiting the testing room
In addition to the above, universal precautions still apply regarding protecting mucosal membranes from pathogens, as well as practicing hand hygiene.
More Information:
Personal Protective Equipment for Infection Control (FDA)
COVID-19: Control and Prevention (OSHA)
COVID-19 PPE for Healthcare Personnel (CDC)
COVID-19: Infection Control
COVID-19: Infection Control Guidance
Questions About Personal Protective Equipment (PPE)
Testing 101
What types of tests are available for SARS-CoV-2?
There are three types of tests currently available for testing:
Molecular Testing
The gold standard of testing: Reverse Transcriptase-Polymerase Chain Reaction or RT-PCR)
Used for diagnostic purposes; uses reverse transcriptase to create and amplify DNA from SARS-Cov-2 RNA
Can determine if there is an active infection (viral DNA/RNA), but not whether a resolved patients previously had the condition (replicating virus or remnants of viral DNA/RNA)
Requires special equipment and/or machinery
Results typically take longer than 45 min and could take up to several hours
False Negatives may occur
Antigen Testing - Antigen Detection
Detects viral proteins in the nasal cavity
As of 5/26/20, there is only one antigen test (Sofia 2 SARS Antigen FIA - lateral flow immunofluorescent sandwich assay) with an EUA, indicated for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in NP and NS swabs added to VTM. It must be used in conjunction with the Sofia 2 Instrument. This test is authorized for high, moderate, or waived complexity labs and for POC. Results are available in 15 minutes.
Rapid results available, but it is not as sensitive as PCR tests and may not detect all active infections (false negatives); negative results from an antigen test may need to be confirmed with a PCR test.
Serological Testing - Antibody Detection
May show past or present infections, but there is not enough evidence to support that the presence of IgG antibodies to COVID-19 provides short- or long-term immunity to illness
Portable devices don’t require bulky equipment for analysis
Rapid results available in 15 minutes
Potential for false negatives and false positives
Are antibody, or serology tests, used to diagnose SARS-CoV-2 infections?
In short, no. Because antibodies are part of the body’s immune response to exposure and not the virus itself, such testing cannot be used for diagnosis of infection based on varying individual responses. Antibody tests are intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection, by detecting antibodies to SARS-CoV-2.
The performance of serology tests is measured by:
Sensitivity - Ability to identify those with antibodies to SARS-CoV-2 (true positive rate)
Specificity - Ability to identify those without antibodies to SARS-CoV-2 (true negative rate)
Serology tests most commonly detect the IgM and IgG antibodies and can help determine if a person or population has developed antibodies indicative of an adaptive immune response.
A serology test can yield a falsely negative result in infected patients (e.g., if the antibody has not yet developed) or a falsely positive result (e.g. if antibodies to a coronavirus type other than the pandemic novel strain are present). Other reasons for a false negative may be due to user error (e.g. not utilizing a buffer if called for) or poor design (due to the viral antigen or antibody they are targeting).
In summary, positive results from an appropriately validated serology test can indicate whether a patient has had recent or prior COVID-19 infection.
What are the recommendations for testing priorities and protocols?
The CDC recommends Molecular or Antigen testing to diagnose acute infections:
High Priority
Hospitalized patients w/ symptoms
Healthcare facility works, workers in congregate living settings, and first responders w/ symptoms
Residents in long-term care facilities or other congregate living settings, including prisons and shelters, with symptoms
Priority
Persons w/ symptoms of potential COVID-19 infection (fever, cough, SOB, chills, muscle pain, new loss of taste or smell, vomiting/diarrhea, and/or sore throat)
Persons w/o symptoms who are prioritized by health departments or clinicians (public health monitoring, sentinel surveillance, screening of other asymptomatic individuals according to state and local plans)
What tests for COVID 19 have received EUA?
Tests that have received an EUA are listed here.
Once a test receives an EUA for use at the point of care, is it considered CLIA waived?
Once a test gains EUA for use at the point of care, it is considered CLIA waived. The EUA Letter of Approval will include approved settings within the designation (e.g, highly complex lab, moderately complex lab, etc). Clinics without a CLIA Waiver will be permitted to run POC tests.
A Few Notes on Clinical Laboratory Improvement Amendments (CLIA)
Under CLIA, a laboratory is defined as a facility that performs testing on materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or assessment of the health of human beings.
You can enroll your lab in the CLIA program by completing the CMS-116 form and sending it to the appropriate State Agency. As of March 26, 2020, the CMS has amended its policies to expedite the application and review process.
Please see below for more helpful links regarding CLIA Certificates of Waiver.
Three CLIA certification complexities may be assigned to diagnostic tests with EUA Designation:
High - stringent regulations regarding testing personnel
Moderate - more stringent than waived or provider-performed microscopy (PPM) labs but less stringent than high complexity labs
Waived - Point-of-Care testing and personnel regulations not as stringent
What is the CLIA categorization of a test that is offered prior to or without an EUA?
If a test is being offered prior to or without an EUA, then that test has not been reviewed yet by the FDA and has not received a CLIA categorization. The FDA is authorizing the use of such tests under a default categorization of high complexity until an EUA is given.
What States have chosen to authorize labs within that State to develop and perform tests for COVID-19?
The following States have notified the FDA that they choose to expedite COVID-19 testing, per the Policy for Coronavirus Disease-2019 Tests. The FDA will not review the processes adopted by each state. States using the process are:
State of Connecticut
State of Maryland
State of Mississippi
State of Nevada
State of New Jersey
State of New York Department of Health, Wadsworth Center
Washington Department of Health
More Information:
FAQs on Testing for SARS-CoV-2 (FDA)
How to Obtain a CLIA Certificate of Waiver (CMS)
CLIA Laboratory Guidances During COVID-19 Public Health Emergency (CMS)
COVID-19 Testing - Guide for Physicians (AAFP)
Interim Guidance: Evaluation and Testing for COVID-19 (CDC)
World Report: Developing Antibody Tests for SARS-CoV-2 (The Lancet, Vol. 395, April 4, 2020)
Video: Coronavirus Antibody Tests - What They Tell Us (JAMA)
Interpreting Diagnostic Tests for SARS-CoV-2 (JAMA, May 6, 2020)
COVID-19 Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients (FDA)
EUA Authorized Serology Test Performance (FDA)
Testing Recommendation for COVID-19 in Patients Planned for Surgery - Continuing the Service and ‘Suppressing’ the Pandemic (British Journal of Oral and Maxillofacial Surgery, April 2020)
Assay Techniques and Test Development for COVID-19 Diagnosis (ACS)
Developing a National Strategy for Serology (Antibody Testing) in the United States (JHU)
Guidance on Interpreting COVID-19 Test Results (Whitehouse & CDC)
Getting Back to Work
Once stay-at-home restrictions are relaxed, physician practices should thoughtfully plan when and how to reopen to full capacity. Remember to comply with local governmental guidance. Some states, counties, and cities have modified or extended stay-at-home orders. Please refer to the Factsheet linked below that details state-specific delays, and when elective or non-urgent procedures may resume.
Below is a brief checklist of considerations when reopening and/or ramping up the clinic:
Make a Plan: Consider a ‘soft reopening’ where you open incrementally
Identify what visits can be done via telehealth and perform those visits remotely.
Direct administrative staff who do not need to be physically present in the office to stay home and work remotely.
Consider a tele-triage program and screening patients before in-person visits
Create a protocol for clinic staff to evaluate the patient’s condition and symptoms. This should assist in determining if the visit is best handled via telemedicine or as an in-person visit.
Before a patient arrives at the office, the practice should verify as best it can that a patient does not have symptoms of COVID-19. In the case of suspected infection and the patient must be seen, flag the patient utilizing EMR features to alert the clinic in advance .
Change the arrival, registration, and processing procedures for patients to reduce the number of patient-staff interactions. Implement online registration (readily available via most EMRs).
Assess PPE needs and alternatives such as cloth masks and replenish as able
Retrain staff on proper donning and disposal of PPE.
If necessary, retrain staff on proper cleaning and disinfecting protocols.
Have supplies delivered in advance before reopening so that sporadic delivers do not disrupt the daily plan.
Clinical staff should wear face masks and gloves when treating non-ill patients because of concerns about asymptomatic COVID-19.
In the case of suspected infection, clinical staff should wear face masks, gowns, eye protection, and gloves.
Institute safety measures for patients and staff
Consider a modified schedule to avoid high volume or density within care rooms or waiting areas.
Operating at capacity will not allow for adequate cleaning and disinfection of patient care rooms and surfaces - consider reduced capacity.
Limit patient companions.
Limit the number of interactions between employees and patients.
Alternate employee shifts so that the same group of employees is not repeatedly exposed to one another.
Restrict the number of patients and companions entering the facility.
Establish a rotation between work from home and onsite shifts.
Lengthen shifts to reduce the number of employee changes per day.
Change operating hours.
Provide and encourage services through online or phone reservations to mitigate in-person interactions.
Ensure and prioritize workplace safety to limit interactions and make the workspace conducive to distancing
Conduct meetings virtually.
If meetings must happen in-person, limit attendance and time frames.
Utilize outdoor spaces to conduct business operations while social distancing.
Place shields or other physical barriers between employees and patients in areas where maintaining a 6’ distance may not be possible.
Provide and utilize masks in close-contact settings.
Place floor markings to direct traffic, ensuring 1-way directional flow in enclosed spaces.
Remove chairs from waiting area and review seating arrangements in the waiting room to allow for distancing.
Communicate personal health requirements clearly to clinicians and staff, including not working if ill.
Adopt a flexible and sick policy to accommodate illness.
Reduce the number of objects being transferred between staff and patients.
Reduce business functions that require domestic travel outside of your community.
If domestic travel is necessary, discourage mass transit use and encourage private transport such as rental cars.
Provide PPE to traveling employees (gloves, masks, etc.).
Enforce Sanitation and Hygiene measures.
Identify priority cleaning supplies to disinfect surfaces, shared equipment, and facilities.
Procure, store, and maintain necessary cleaning supplies, PPE, and other critical supplies (face masks, hand sanitizers, tissues, and other paper products).
Request an increase in supplies from your distributors.
If necessary, identify alternative distributors or manufacturers.
Maintain handwashing and hand sanitizer stations throughout the facility with clear signage and notices.
Develop and implement a sanitation plan with increased cleaning schedules for surfaces, equipment, and rooms.
Identify who is responsible for cleaning and ensure those in charge of cleaning are provided with PPE and supplies.
Allow ample time for cleaning and disinfection of surfaces.
Continue to communicate system changes and policy updates with staff via a weekly newsletter or group call.
More Information:
Checklist to Prepare Physician Offices for COVID-19 (AAFP)
Opening Up America Again: Reopening Facilities to Provide Non-Emergent Non-COVID-19 Healthcare (CMS)
A Physician Practice Guide to Reopening (AMA)
Factsheet: State Action related to Delay and Resumption of ‘Elective’ Procedures During COVID-19 Pandemic (AMA)
Considerations on Re-Opening Your Practice (AAFP)
Guidance for Cleaning and Disinfecting (CDC)
COVID-19 CDC/EPA Cleaning & Disinfecting Guidances (CDC)
6 Steps for Safe & Effective Disinfectant Use (EPA)
COVID-19: Using PPE (CDC)
Factsheet for Healthcare Personnel: N95 Respirators and Decontamination (FDA)
COVID-19 Coding and Billing Guidelines
On April 10, 202, the American Medical Association (AMA) announced new CPT codes for reporting and tracking of tests specifically for COVID-19.
CPT Codes:
86318 - Immunoassay for infectious agent, antibody(ies), qualitative or semiquantitative, single-step method (e.g., reagent strip)
86328 - Child code to 86318 specific for testing SARS-CoV-2 or COVID-19
ICD-10 Codes:
U07.1 - to document a positive COVID-19 test result (presumptive positive test results should be coded as confirmed)
More Information:
CPT Assistant: SARS CoV-2 Serologic Laboratory Testing (AMA)
ICD-10-CM Official Coding and Reporting Guidelines, 4/1/2020-9/30/2020 (CDC)