PRODUCT BROCHURE
PRODUCT VIDEO
RESOURCES
Demystifying Quality Control in Orthobiologics
FREQUENTLY ASKED QUESTIONS
-
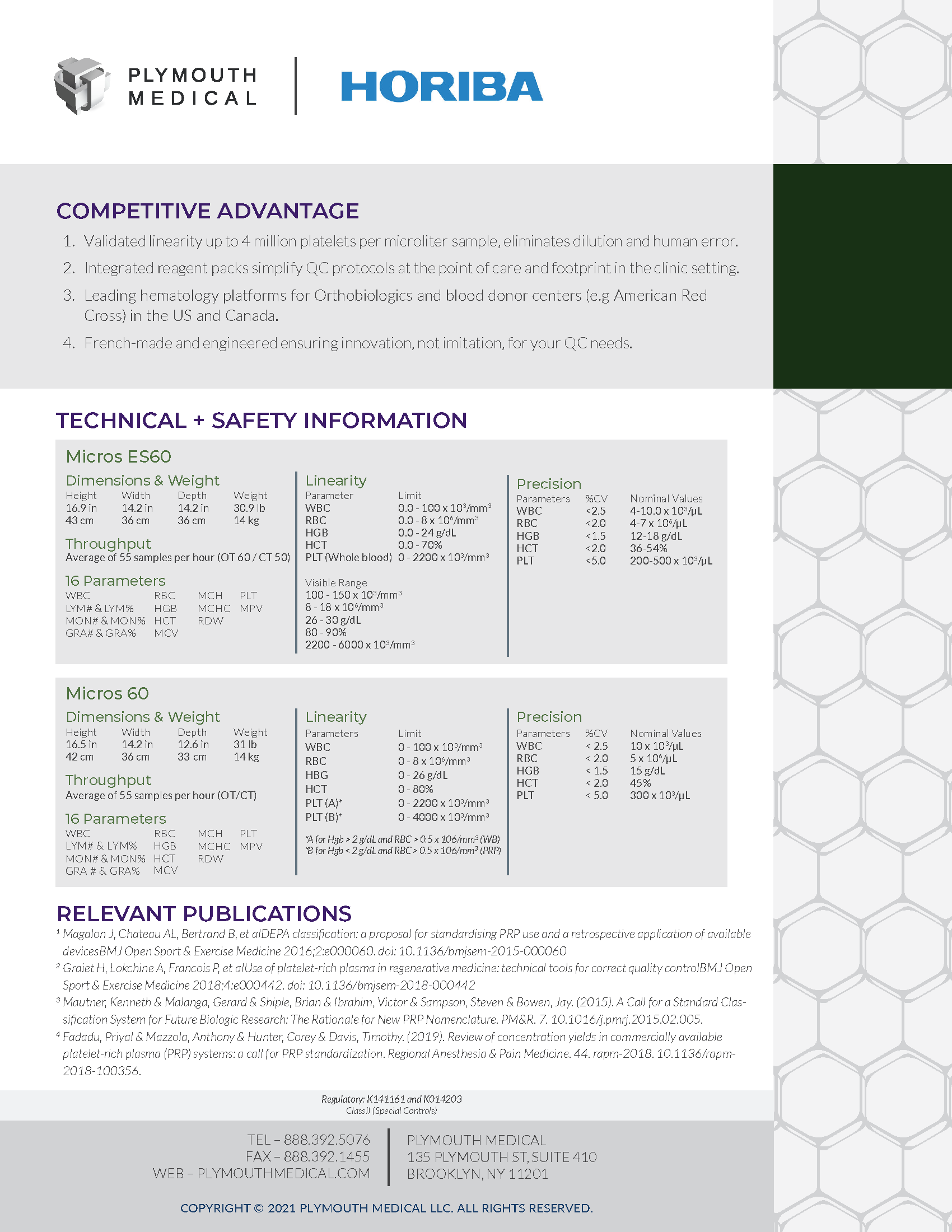
The Micros 60 and Micros ES60 devices are both 3 part DIFF Hematology Analyzers, universally recognized for reliability, high platelet range and ease of use. These devices characterize whole blood and Platelet Rich Plasma (PRP) in less than 1 minute, and can even be used to detect red blood cells in other tissues such as synovial fluid (off-label use). The main difference between the two models is that the Micros 60 has been validated and cleared by the FDA to have a higher platelet range. In fact, the Micros 60 hematology analyzer is the only device to have been FDA cleared to assess PLT counts to 4 million PLT/µL eliminating the need to dilute PRP samples for faster and more accurate testing at the point-of-care. In addition, whereas the Micros ES60 Hematology Analyzer has a touchscreen, integrated thermal ticket printer and barcode reader, the Micros 60 Hematology Analyzer comes with an external printer. The LiteDMv3 Data Manager laptop, which captures and stores patient cell data, is optional and can be purchased separately.
-
The overall difference between the bottle and minipack model is that the minipack system is an enclosed box which contains all three needed reagents (ABX MINIDIL, ABX MINICLEAN and ABX ALPHALYSE) and also acts as a bladder for the system. This simplifies cell counting at the point of care as it removes the need for cumbersome reagent bottles (#3 separate products) and access to a sink. There is no difference in terms of device or disposable pricing. Nearly all orthobiologic clinics and all of Plymouth Medical clients use the Minipack Reagent system. All in all, the minipack system is easier to use and more suitable for clinics that do not analyze a high volume of samples, such as daily and frequent CBC analyses.
-
Both the Micros 60 and Micros ES60 are manufactured in France.
-
Yes, the Horiba Hematology analyzers are FDA cleared. In fact, the Micros 60 is the only device to have been FDA cleared to assess PLT counts up to 4 million PLT/µL which are optimal levels for PRP platelet counts (The Micros ES60 is FDA cleared up to 2 million PLT/µL). Due to this precise sensitivity, samples do not require dilution for accurate testing at the point-of-care.
-
The Horiba Micros ABX cell counters have a 1 year warranty which includes any on-site service calls and replacement parts free of charge. If the Horiba Field Technician is unable to fix your device, you will receive a new device at no cost during your warranty period. Please reach out to our Customer Service Team via email at customerservice@plymouthmedical.com or call (888) 392-5076 Ext.2 for more information. Unlimited and complimentary technical support is available through the Horiba Technical Support Team at (888) 903-5001 Ext 3 during and after your warranty period.
-
For years 2-5, you can opt-in to a limited service agreement. The service agreement can be reviewed annually. Plymouth Medical has negotiated special pricing for the service agreement: $2000/year (Regular Pricing: $3600/year). You can decide whether you would like to opt in to the limited service agreement at the end of your warranty period. The limited service agreement includes ONE (1) annual on-site preventative maintenance (PM) and ONE (1) on-site service call in the event that you are unable to troubleshoot your device with the Horiba Technical Support via phone; Additional service calls at a flat fee of $1000 and capped at $4000 annually. Any service arrangement will be made directly with Horiba. Please reach out to Customer Service via email at customerservice@plymouthmedical.com or call (888) 392-5076 Ext.2. for more information regarding the service agreement. Unlimited and complimentary technical support by phone is available through the Horiba Technical Support Team at (888) 903-5001 Ext 3 with or without the limited service agreement.
-
You will only need to run linearity if you are planning to bill Medicare for CBCs. Since you are using the cell counter for quality control of PRP and/or research purposes, you will not need to purchase the Linearity Kit. Should the Horiba Field Technician mention the Linearity Kit, let them know that you do not need the Linearity Kit as your office will not use the cell counter as a diagnostic device or bill Medicare or commercial insurances for CBCs.
-
Micros 60: The Micros 60 tests for #16 parameters: WBC, LYM#, LYM%, MON#, MON%, GRA#, GRA%, RBC, HGB, HCT, MCV, MCH, MCHC, RDW, PLT, MPV
Micros ES60: The Micros ES60 tests for #16 parameters: WBC, LYM#, LYM%, MON#, MON%, GRA#, GRA%, RBC, HGB, HCT, MCV, MCH, MCHC, RDW, PLT, MPV
-
There is no specific certification required for operating the device however it is strongly recommended that the operator is present during the Horiba Field Technician’s training. The device is used off-label to characterize PRP and other orthobiologics for research purposes. If used for diagnostic purposes, this device is cleared to run in a moderately complex lab as designated by CLIA. For Orthobiologics, this device is used primarily for quality control and research as patient results are not billed for or used to diagnose a new or existing condition.
-
Yes, the cell counters can operate above 6K ft altitude. However, the Horiba Field Technician will make corrections to the system’s bubbling and pressure during initial setup. This process will compensate for the way high altitudes alter how a sample is pulled into the system.
-
Yes, but Synovial Fluid is off label use and is very sticky which can cause maintenance issues if you do not properly clean the device afterward.
-
No, your device will be installed by a Horiba Field Technician who will come to your clinic on an agreed installation date. The actual installation time of the device, which does not require clinic attention, is approximately two hours. This will include unboxing, placement of the device, initial user set-up and initial calibration.
-
You will receive on-site training from a licensed Horiba Field Technician along with a HORIBA portal with videos and quizzes. We highly recommend that the appropriate staff be present for the installment/training date for which you should block off half a day (approx. 4 hours depending on how many questions are asked). Please note that the training will not be recorded for future use. We will provide you with everything that is needed for the on-site training. Once your device has shipped, Plymouth Medical will also provide you with additional training resources.
-
In order to run samples with the cell counter, you will need Minotrol Controls for Quality Control (QC), Minipacks (reagents) and BD K2 EDTA Microtainers. We also recommend to frequently clean the cell counter with the Minoclair and calibrate the device every 6 months or as needed with the Minocal. Please review our disposables summary on our Horiba resources page for additional information on the disposables. For pricing, please contact our Customer Service Team customerservice@plymouthmedical.com or (888) 392-5076 Ext.2.
-
The Minotrol Controls for QC have a shelf life of 2-8 weeks. The controls you will receive with the Starter Kit have a shelf life of 2 weeks (opened or unopened). The bi-monthly controls that will autoship to you have a shelf life of 2 months (opened or unopened). The Minipack reagent has a shelf life of approximately 9 months unopened (3 months once opened or the expiration date has reached whichever comes first). The BD K2 EDTA Microtainers have a shelf life of approximately 2 years. The Minoclair cleaning solution has a shelf life of approximately 9 months when unopened (6 months once opened or the expiration date has been reached, whichever comes first). The shelf life of the Minocal calibrator is 2-3 weeks (opened or unopened). Once opened the vial itself is only good for 24 hours.
-
You can order your disposables by emailing customerservice@plymouthmedical.com or calling our customer service hotline (888) 392-5076 Ext.2. The turnaround time for disposable orders is 24-48 hours.
-
Since the Minotrol controls need to be run every day you plan on testing samples, the LOW, NORMAL and HIGH controls are auto-shipped to you every two months. It does not matter whether you use the cell counter 1 time or 100 times; the controls have a set expiration date. You can find the auto-shipment schedule and QC data for the Minotrol controls on our resource page: https://www.plymouthmedical.com/horiba-resources.
-
The manuals for the Micros 60 and Micros ES60 can be found on our resources page: https://www.plymouthmedical.com/horiba-resources
-
Yes, we recommend using the K2 EDTA microtainers for both whole blood and PRP samples. Studies on accurate cell counting for PRP samples point to K2 EDTA microtainers providing the best stability and helping with platelet aggregation regardless of what anticoagulant you are using for your PRP preparation. Your initial instrument order will include at least #1 pack of 50 K2EDTA (500µl=0.5 mL) microtainers. Replenishment orders can be placed with our customer service team customerservice@plymouthemdical.com or (888) 392-5076 Ext.2. The turnaround time for disposable orders is 24-48 hours. For more information on best practices regarding quality control of platelet-rich plasma products, please refer to this peer-reviewed article by Hajer Graiet et al.
-
Reagent waste may be placed in a red biohazard container for disposal. It does not need to be puncture-proof, just lined as biological waste.
-
Yes, we recommend to deep clean the device at the end of each testing day to ensure the reagent lines remain clear and to prevent protein/cellular build up. For each cleaning cycle, you will need 6mL of Minoclair. Cleaning instructions can be found in the manuals which can be accessed on our resources page.
-
You will need approximately 2 drops (100µL) to run an analysis. The probe will then use 10µL of the sample for each testing cycle. Before adding the sample to the microtainer, ensure the sample is well suspended by gently agitating the syringe or other holding vessel.
-
No, you have up to 24 hours to run the analysis if you use a K2 EDTA microtainer as the collection container. K2 EDTA are microtainers coated with anticoagulant which are able to give stable platelet counts for whole blood and PRP up to 24 hours. For more information please refer to this peer-reviewed article by Hajer Graiet et al.
-
Upon daily start-up, you will run the LOW, NORMAL and HIGH quality controls using Minotrol QC material to ensure results are accurate. Instructions on how to run QC can be found in the manual and our Quick Guide which can be accessed on our resources page. Once you have run your daily LOW, NORMAL and HIGH quality controls, it is not required to rerun the QC if a full shutdown and restart is done. However, please check with your clinic coordinator on protocols for running a QC. For example, low-volume testing clinics may run a QC every day of testing while hospitals tend to run one or more QC checks per shift. Your clinic’s testing protocols will ultimately dictate when a QC run is necessary.
-
No, you will only need to run a quality control test if you plan on analyzing samples for that day. If you are using the device infrequently, it is recommended to perform a startup cycle once per week if the device has not been used for testing.
-
No, you will only have to run #1 test for your whole blood sample and one test for your PRP sample. The quality control test that is done when you turn on the device will ensure the accuracy of your test results. If you are collecting data as part of an IRB-approved study, then your institution's protocol may necessitate performing multiple analyses on a single sample - check with your research coordinator on how many runs are to be performed.
-
You will need to change the minipack after approximately 45 patients (assuming you are running two tests per patient). One minipack has 150 cycles. For each QC, three cycles are run. As an example, if you run your device Mon-Fri for 4 weeks, you will use up 60 cycles for QC leaving you with 90 cycles. For each patient, you will need to run two separate cycles: One (1) for Whole Blood (i.e baseline) and One (1) for PRP - 90 cycles / 2 = 45 patients. The manual and our Quick Guide (see resource page) show how you can check for the remaining cycles for the minipack.
-
The manufacturer recommends calibrating the device every 6 months with the Minocal, or as needed. The manuals (see resource page) include detailed instructions on the calibration of the device. The calibration takes about 30 minutes. Calibration is an exceptional procedure which must be carried out in case of certain technical interventions (installation, maintenance or service intervention). If you opted into the limited service agreement, the calibration will be done during your complimentary annual on-site preventative maintenance (PM). The calibration should not be carried out to compensate for drift on a result due, for example, to a clogging of the instrument. Contact Horiba Technical Support at (888) 903-5001 Ext 3 to determine if recalibration of the device is appropriate for a specific problem.
-
Common errors that happen include that the device does not process a sample, the probe does not descend properly or is stuck in down position, the microtainer is stuck, incorrect/odd result are reported, , the reagents are expired or empty, there is not enough blood in the vial/tube, proper cleaning was not followed causing erroneous results, and specimen were not mixed properly prior to running. A list of common errors and how to resolve them can be found in our Quick Guide (see resource page).
-
The LiteDMv3 Data Manager is an optional patient data management software that enables you to export results and demographics to an EMR via an HL7 bridge. The software can be purchased separately for both the Micros 60 and Micros ES60. More info here. The LiteDMv3 Data Manager software purchase includes a Lenovo laptop.
-
There are periodic LiteDMv3 Data Manager software updates but they are not required. So you will be able to continue using the cell counter without making any updates to your system. The LiteDMv3 Data Manager software updates can only be installed during an on site service call or preventative maintenance (PM) which is included with the limited service agreement.
-
The laptop has the ability to download previous results to a thumb drive for additional storage.
-
The laptop with the LiteDMv3 Data Manager software comes with the Windows 10 Pro operating system.
-
Yes, the Horiba Field Technician will install the LiteDMv3 Data Manager software system and also provide you with step by step instructions on how to use the software system during your training.
-
Yes, you can connect the LiteDMv3 Data Manager software to an EMR via an HL7 bridge. Existing EMR interfaces include: Athena, Flatiron, Epic, eClinicalWorks, and CureMD.
-
Micros 60: Open Tube: 60, Closed Tube: 55
Micros ES60: Open Tube: 60, Closed Tube: 50
-
Micros 60: Height: 16.5 in (42 cm), Width: 14 in (36 cm), Depth: 12.5 in (33 cm)
Micros ES60: Height: 16.9 in (43 cm), Width: 14.2 in (36 cm), Depth: 14.2 in (36 cm)
-
Micros 60: 31.0 lbs (14 kg)
Micros ES60: 35.7 lbs (17 kg)
-
The Micros 60: Retains the last patient data run on the machine. The LiteDMv3 Data Manager that comes with the Micros 60 expands the patient result storage capabilities as the application runs on a laptop with greater memory.
The Micros ES60: Stores up to 1000 patients results with histograms.
-
Micros 60: The printer that is included with the Micros 60 is a Brother Laser Printer. The part numbers for the toner and drums can be found on a sticker on the top of the printer as well as in the manual that comes with the printer.
Micros ES 60: The Micros ES60 comes with an integrated thermal ticket printer (no external printer).
-
Yes, the Horiba Micros cell counters have the capability to process, store or transmit PHI.
-
Yes, the Horiba Micros ABX cell counters have the capability to connect to your or any network including wireless. The only reason network connectivity would be needed is for an EMR interface, otherwise it would just be used for remote access if granted.
-
No remote computer/network access to the equipment/software by outside personnel is required though it is preferred for better tech support.
-
Yes, the Horiba Micros ABX cell counters can be patched to fix security vulnerabilities.
-
Yes, the Horiba Micros ABX cell counters have the ability to encrypt data.
-
Yes, the Horiba Micros ABX cell counters support anti-virus and/or whitelisting.
-
Yes, administrative or support passwords can be changed from the default in the Setup Menu. Please review the manuals on our resource page for instructions.