QUALITY CONTROL IN ORTHOBIOLOGICS: CELL COUNTING AND REGISTRIES
When we founded PLYMOUTH MEDICAL 8 years ago, there wasn’t any clear consensus on the use of autologous biologics for the various MSK or aesthetic indications for which PRP or autologous stem cell therapies were being injected. Unfortunately, there isn’t a great deal more consensus today.
Most of the journal articles we have read have fundamental shortcomings in sample size and lacking cellular characterization of PRP and stem cell therapies; this exacerbates the confusion related to autologous biologics and further leads to conflicting findings. Various Orthobiologics scientists and providers have since called for the classification of injectates to establish better correlations to pain and functional outcomes. Since each patient and commercial kit present inherent variability, most understood that better categorization regarding Dose, system Efficiency, cellular Purity (in terms of RBC and WBC contamination) as well as Activation was needed. Mautner et al. (2015) concluded that “widespread adoption of these [classification] recommendations will facilitate interpretation and comparison of clinical studies and promote scientifically based progress in the field of regenerative medicine”.
We felt the same way and soon set out to provide our clients with the tools necessary to define and determine the cellular composition of their autologous injectates at the point of care; in 2016, we introduced the Horiba Micros 60 and Chemometec NC-200 which are best in class in terms of cost, ease of use and accuracy. N.B: PLYMOUTH MEDICAL also has nothing to hide regarding the cellular composition of our EmCyte PRP, Bone Marrow and Plasma concentrates.
This is a short introduction video we have created to help regenerative practitioners understand Quality Control in autologous biologics at the point of care. We hope it sheds more light on the possibilities and look forward to hearing from those of you that are interested to learn more by scheduling a call with us or emailing us at info@plymouthmedical.com.
While quality control and testing takes time, dedication and resource, the future of Orthobiologics depends on a much deeper understanding of what is being injected; case in point, just take a look at the debacle currently unraveling for mostly unproven and ill-defined perinatal products. Most providers involved in Regenerative Medicine are left to hope the data their sales reps or manufacturers present them is accurate and most simply don’t have the means or resources for cellular assessments in this competitive market.
In the spirit of moving the science forward and to advance the field of Orthobiologics, PLYMOUTH MEDICAL has supported our clients to test their injectates, follow up with patients and use bespoke registries to measure outcomes more efficiently such as DataBiologics. The DataBiologics patient outcome registry allows our providers to track the safety and efficacy of Orthobiologic treatments with easy patient enrollment and automatic survey assignment. PLYMOUTH MEDICAL clients receive complimentary onboarding to start collecting real-world data for better patient care. Contact us for more info on DataBiologics.
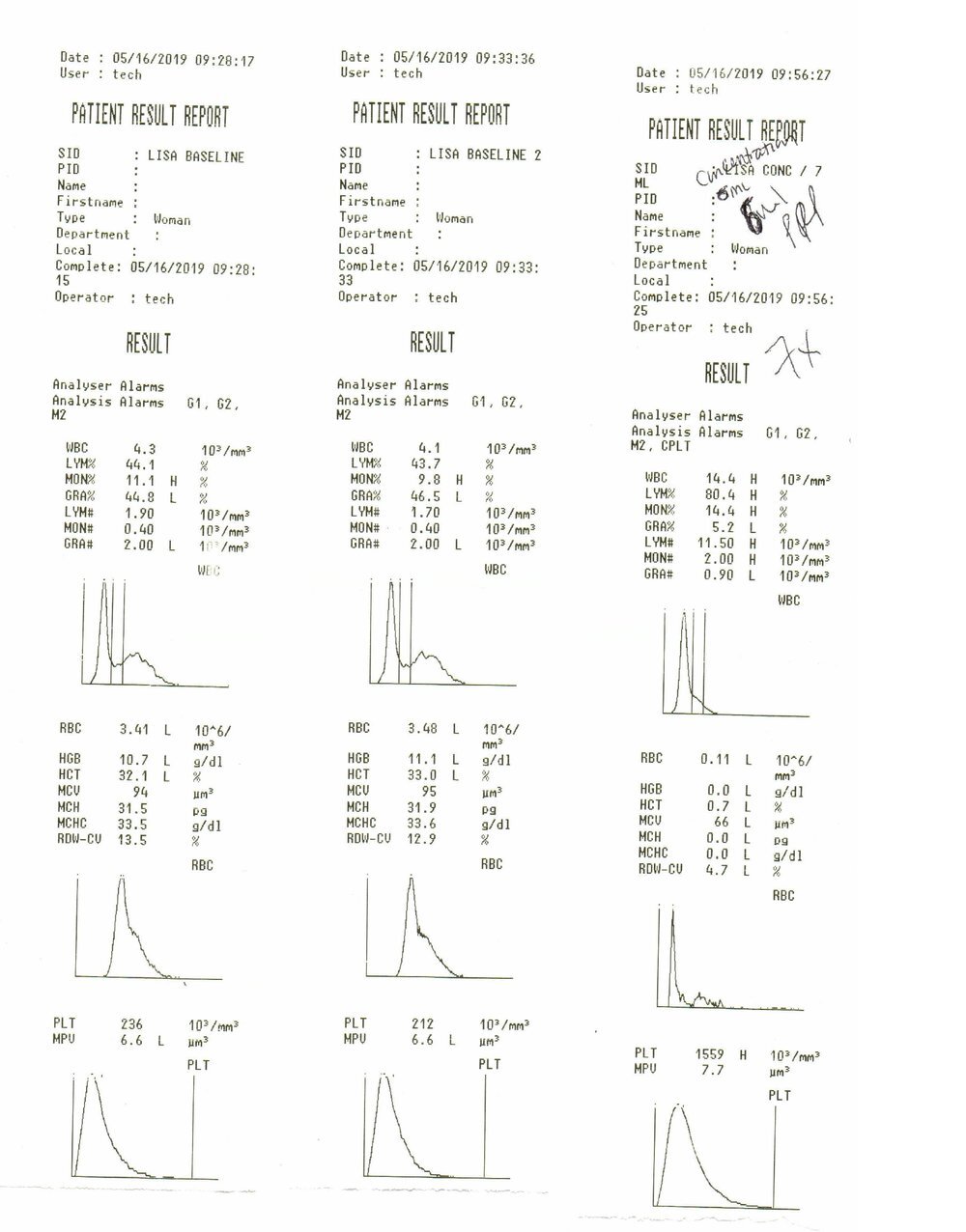
Here are the printout results from our hematology experiment; left two data sets are baseline and the rightmost data set are the results generated from our 6mL PurePRP. Total dose of platelets delivered was therefore 9.35 billion platelets, platelet recovery was 78%, purity was good with 0.7% hematocrit and a reduction in granulocytic white cells. This PRP was not activated prior to injection in the patient.
MICROS 60 CLOSED SYSTEM
07/2019