The Menopause Edition
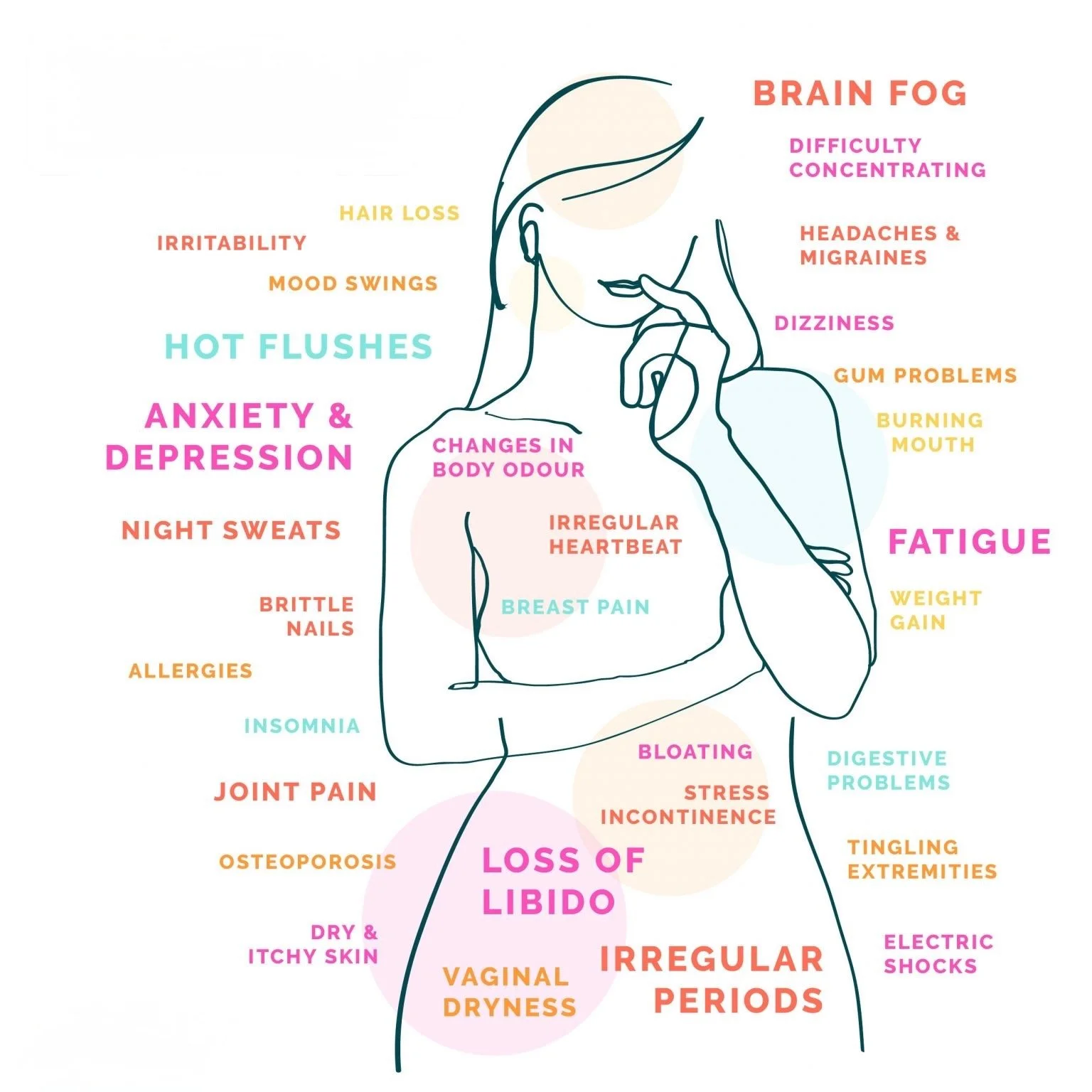
Menopause is a natural milestone, but for many women, it comes with disruptive symptoms that affect quality of life. From genitourinary syndrome of menopause (GSM) and sexual dysfunction to urinary incontinence and visible skin changes, these challenges are common—and often under-addressed.
While hormone replacement therapy (HRT) remains the gold standard, not all women can—or want to—use hormones. This has driven growing interest in non-hormonal, regenerative solutions. Platelet-rich plasma (PRP), leveraging the body’s own growth factors, is emerging as a minimally invasive option with promising applications in women’s health.
We reviewed the role of biologics in managing these symptoms and developed a treatment guide that aligns current clinical evidence with practical applications. Although this overview is preliminary, the evidence is clear: high-quality PRP is emerging as a highly versatile modality and may represent a valuable addition to modern therapeutic strategies.
Urogynecologic Applications of PRP
Menopause can lead to changes in vaginal, sexual, and urinary function that impact quality of life. PRP is a minimally invasive therapy that leverages autologous growth factors to support tissue repair and improve these functional outcomes.
Genitourinary Syndrome of Menopause (GSM): Estrogen deficiency during menopause contributes to vaginal thinning, dryness, irritation, and dyspareunia. PRP has been investigated as a regenerative therapy for GSM, with mechanisms including enhanced mucosal thickness, vascularity, and lubrication.
Clinical studies report meaningful improvements in vaginal health and patient-reported outcomes. In a 2025 randomized clinical trial, PRP demonstrated significant efficacy compared with topical estrogen among women with vulvovaginal atrophy who were non-responders to hormone therapy, highlighting its potential as a non-hormonal option for refractory cases. [1] Similarly, a pilot study by Saleh et al. showed that autologous PRP injections were safe, well-tolerated, and associated with improvements in vaginal health scores, dryness, irritation, and sexual discomfort, with no reported adverse effects. [2]
Sexual Function: Sexual function often declines during menopause due to hormonal changes, tissue thinning, reduced vascularization, and genitourinary discomfort. These changes can lead to diminished sensitivity, arousal difficulties, dyspareunia, and reduced orgasmic capacity.
PRP may help to counteract these changes by enhancing blood flow, stimulating collagen production, and supporting nerve regeneration in the vaginal and clitoral regions. In a retrospective study of postmenopausal women with sexual dysfunction, PRP injections into the anterior vaginal wall significantly improved Female Sexual Function Index (FSFI) scores, particularly within the orgasm domain (p < 0.001), alongside improvements in genital self-image and sexual distress. [3]
A systematic review further supports these findings, noting consistent improvements in FSFI, Vaginal Health Index (VHI), and Female Sexual Distress Scale (FSDS) following PRP protocols involving monthly injections over three months. [4]
Stress Urinary Incontinence: Stress urinary incontinence is a common concern in postmenopausal women, often resulting from estrogen deficiency, pelvic floor weakening, and reduced urethral sphincter support. These changes can contribute to leakage with coughing, laughing, or physical exertion, impacting both daily functioning and quality of life.
Periurethral PRP injections have shown promise as a regenerative therapy by stimulating collagen synthesis, angiogenesis, and tissue remodeling in the urethral sphincter complex. A prospective pilot study in women with mild-to-moderate SUI demonstrated significant reductions in pad use and leakage episodes following periurethral PRP, with improvements maintained over a 12-month follow-up period. [5]
More recently, a randomized controlled trial compared PRP with saline injections and found that PRP-treated women experienced greater improvements in subjective continence measures and quality-of-life scores, further supporting its therapeutic potential in this population. [6]
Esthetic Applications of PRP
Beyond urogenital health, menopause often accelerates aging of the skin and hair due to declining estrogen levels. PRP has become a sought-after modality in aesthetic medicine for addressing these changes by stimulating the body’s natural regenerative processes. Through targeted injections, PRP can improve skin texture, tone, and elasticity, while also promoting hair follicle health and density, offering minimally invasive options for facial rejuvenation and hair restoration.
Facial Rejuvenation: Menopause often accelerates visible signs of aging, including fine lines, decreased skin elasticity, and thinning. PRP leverages the body’s growth factors to stimulate collagen production, improving skin texture, tone, and elasticity—offering a natural, minimally invasive option for postmenopausal facial rejuvenation. This regenerative approach offers a natural solution for postmenopausal women seeking to rejuvenate their facial appearance.
For a comprehensive understanding of how PRP can aid in facial rejuvenation, please refer to our detailed blog on Esthetic Applications Using Platelet Rich Plasma.
Hair Restoration: Menopausal hair thinning is a common issue, often linked to hormonal changes and reduced follicular activity. PRP injections into the scalp have been shown to promote angiogenesis, stimulate hair follicle regeneration, and increase hair density and thickness. This minimally invasive treatment provides a hormone-free alternative for managing menopausal hair loss, offering potential benefits for women experiencing this condition.
To explore how PRP can support hair restoration, visit our in-depth discussion in the blog on Esthetic Applications Using Platelet Rich Plasma.
Conclusion
Menopause is a multidimensional transition that affects urogenital, sexual, urinary, and aesthetic health, often diminishing quality of life for a large portion of the aging female population. Although hormone replacement therapy remains the standard of care, it is not universally suitable—contraindications, patient preference, and incomplete symptom relief leave many women searching for effective alternatives.
Platelet-rich plasma (PRP) represents an innovative, non-hormonal, autologous therapy that leverages the body’s own growth factors to promote tissue regeneration. Evidence from pilot studies, retrospective reviews, and randomized controlled trials supports its ability to:
Restore vaginal health and improve symptoms of GSM.
Enhance sexual function, particularly orgasmic response and sexual satisfaction.
Improve continence in patients with mild-to-moderate stress urinary incontinence.
Support aesthetic outcomes through collagen induction, skin rejuvenation, and hair restoration.
From a clinical standpoint, PRP offers several advantages: it is minimally invasive, well-tolerated, associated with few adverse effects, and avoids systemic hormone exposure. These features make it an appealing option for women who cannot use HRT or who prefer regenerative, biologically based therapies.
Looking ahead, PRP may integrate into multimodal menopause management, complementing established approaches such as lifestyle interventions, pelvic floor therapy, and selective pharmacologic treatments. Standardization of protocols, dosing, and treatment intervals will be critical as larger, multicenter trials continue to build the evidence base.
For clinicians, understanding the expanding role of PRP in women’s health is essential—not only to remain informed of emerging therapeutic options but also to address the growing patient demand for safe, effective, and natural alternatives during the menopausal transition and beyond.
PLYMOUTH MEDICAL’s Value Added Resources
While the science is promising, clinical outcomes depend on standardization, quality, and education. That’s where PLYMOUTH MEDICAL delivers unmatched value:
✅ Standardized PRP Systems: We provide technology and protocols that ensure consistent platelet concentration, purity, and dosing—removing the variability that limits outcomes.
✅ Evidence-Informed Protocols: Our clinical frameworks align with the latest regenerative gynecology data, giving providers confidence in treatment reproducibility.
✅ Comprehensive Training & Support: From hands-on education to case-based guidance, we empower clinicians to integrate PRP into menopause care effectively and safely.
Citations
08/26/2025